Drug Pricing Negotiations – Are You Ready?
Download a PDF of this articleMark your calendars! Earlier this month, the Center for Medicare and Medicaid Services (CMS) unveiled its timeline1 for implementing the drug price negotiation provision of the Inflation Reduction Act (IRA)2 – sweeping legislation that includes several measures the U.S. government believes will lower drug costs.
The process is already underway as CMS is determining how to identify the top 10 high-spend drugs in Medicare that will be subject to price negotiations with the first wave of drugs selected to be announced on September 1, 2023.
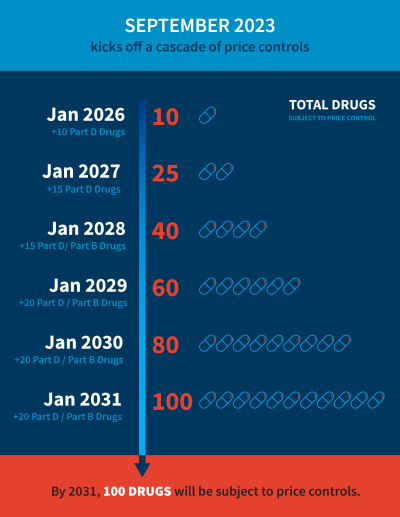
After that, the number of drugs subject to price controls will increase starting in 2026, after the first round of negotiation. Looking at the longer term, the Department of Health and Human Services (HHS) will apply negotiated prices for 10 eligible Medicare Part D drugs in 2026. In 2027, an additional 15 Part D drugs will join the list. Fifteen more Part B or Part D drugs in 2028. And it keeps going in subsequent years.1
Academics, policy experts, and the media have speculated3 which products will make the first list—however, HHS has yet to disclose specific details of the formula they will use to determine how drugs are selected. The data collection process will begin in the coming months. While there is not a definition for “high-cost”4 products, we know that “high-price” Medicare products between June of 2022 and May of 2023 are potential candidates for selection.5
Biopharmaceutical research companies are staring at current and future innovation and treatment uncertainty. But, their standard approach to this challenge is stale. Traditional “R&D is at risk” messaging from innovator companies or trade associations to fend off pricing regulation will not convince supportive stakeholders and critics alike. They have seen that approach many times.
Instead, addressing key business and policy issues now can help chart a forward path for sustainable drug development and credibility when navigating the law’s implementation. Some of those issues include:
- Understanding how “maximum fair price” is determined;
- Ensuring stakeholders understand the law’s implications on price, especially if higher list prices at launch become the norm;
- Anticipating whether or not the definition of value has to change; and
- Considering what business and clinical development solutions can support a sustainable, trusted innovation
Readiness will be key, and there are some fundamental steps companies should consider as they prepare.
1. Leave No Idea on The Table With A Cross-Functional Team
Though many companies have already announced6 the IRA will cause changes to their business strategy, the implications of the law will not uniformly affect all companies. The law’s impact will differ based on companies’ pipelines, portfolios, and reimbursement strategies. Knowing how the law will impact your firm will require a cross-functional team of financial forecasters, pricing and reimbursement specialists, patient engagement teams, brand marketers, legal professionals, and R&D leaders. Most importantly, this team should leave no idea on the table.
“The law’s impact will differ based on companies’ pipelines, portfolios, and reimbursement strategies. Knowing how the law will impact your firm will require a cross-functional team.”
2. Engage In the Process: No One Can Speak Better For Your Company Than You
CMS’s latest communications about the IRA announced public comment periods that go beyond7 what the statute requires. This will give stakeholders multiple opportunities to engage with CMS. In the spring, CMS will issue guidance for the negotiation process and invite public comment on the offer and counteroffer process between Medicare and manufacturers and the methodology for applying maximum fair prices.8
Though trade associations like Pharmaceutical Research and Manufacturers of America (PhRMA) and Biotechnology Innovation Organization (BIO) will likely engage with CMS, individual companies, especially those with narrow portfolios or pipelines, should identify their specific concerns with the IRA process or areas of the law they support. This is an opportunity for companies not just to highlight their response, but to also publicize their structure, mission, and growth areas to CMS.
Having a demonstrated track record of being an active partner and stakeholder will support long-term relationship- building with regulators and lawmakers. CMS will release revised guidance based on public input this summer.9 Start developing your company’s responses early and ensure that input is sought from across the organization.
3. Prepare Your Stakeholders
Patients, advocates, and the medical community have a stake in the outcomes. They will look to companies’ positions and responses as predictors of future business and pipeline decisions because their stakeholders rely on innovative medicines. Companies can deploy proactive and reactive strategies to address their community’s response surrounding upcoming policy or commercial milestones related to the implementation. For instance, surveying individual stakeholders about their perceptions and points of view on the law’s impact provides data for crafting an appropriate outreach and communications strategy. And when policy or business decisions are made and executed, having conversations with core stakeholders, including the advocacy community, ensures a sense of engagement. Though there will likely be points of disagreement, regular and open communication is vital to keeping those relationships healthy.
Surveying individual stakeholders about their perceptions and points of view on the law’s impact provides data for crafting an appropriate outreach and communications strategy.
4. Tell Your Story to Decision-Makers
Although CMS is running the bulk of the show, engagement with Congressional lawmakers matters too. Right now, the drug price negotiation provisions only impact a few products within Medicare, but many Democrats suspect that the measure represents a first step10 towards expanding drug pricing reforms to the private sector in the future. Sen. Bernie Sanders (I-VT), leader of the Health Education, Labor, and Pensions committee, said he plans to move “aggressively on drug pricing”11 in the coming year. Meanwhile, GOP members will likely attempt to challenge the provisions using oversight and investigation tools. Price controls will remain a relevant political topic for the foreseeable future. Ensure policy decision-makers understand the implication for your business and the patient community.
Price controls will remain a relevant political topic for the foreseeable future. Ensure policy decision-makers understand the implication for your business and the patient community.
The Bottom Line
The IRA introduces groundbreaking changes to the U.S. drug pricing landscape, particularly for drug researchers and innovators. But the law’s real impact is still in development as CMS works on implementation details. Specifics about which drugs will be subject to negotiation, how much they will cost, and how the industry will respond are still up in the air. Companies navigating this complex regulatory process will require a thorough strategy and transparent communications.
1 “Medicare Drug Price Negotiation Program – Drug Price Negotiation Timeline for 2026,” Centers for Medicare & Medicaid Services (last accessed January 20, 2023), https://www. cms.gov/files/document/drug-price-negotiation-timeline-2026.pdf.
2 Katie Brown and Allyson Y. Schwartz, “Bracing for Impact: How the Inflation Reduction Act Will Reshape the U.S. Healthcare Landscape,” FTI Consulting Strategic Communications (September 14, 2022), https://fticommunications.com/bracing-for-impact-how-the-inflation-reduction-act-will-reshape-the-u-s-healthcare-landscape/.
3 Micah Johnson, Rahul Nayak, and Sanjay Kishore, “Which Drug Prices Will Medicare Negotiate First? A Physicians’ Perspective,” Health Affairs (September 30, 2022), https://www. healthaffairs.org/content/forefront/which-drug-prices-medicare-negotiate-first-physicians-perspective.
4 Inflation Reduction Act of 2022, H.R. 5376, 117th Congress, Section 1101 Part E (1st Session 2021), https://www.democrats.senate.gov/imo/media/doc/inflation_reduction_act_ of_2022.pdf.
5 Inflation Reduction Act of 2022, H.R. 5376, 117th Congress, Section 1191, Part D (1st Session 2021), https://www.democrats.senate.gov/imo/media/doc/inflation_reduction_act_ of_2022.pdf.
6 Christopher Newman, “Pharma earnings outline drug law’s looming impact on sales, development,” Biopharma Dive, Industry Dive (November 9, 2022), https://www. biopharmadive.com/news/pharma-drug-pricing-ira-law-impact-negotiation/636110/.
7 Rachel Cohrs, “HHS will seek more input on its new Medicare drug price negotiations,” Stat (January 11, 2023), https://www.statnews.com/2023/01/11/hhs-will-seek-more-input- on-its-new-medicare-drug-price-negotiations/.
8 “Key Dates and Estimated Timeline to Implement the Medicare Drug Price Negotiation Program for Initial Price Applicability Year 2026” Center for Medicare & Medicaid Services (Last accessed January 20,2023), https://www.cms.gov/files/document/medicare-drug-price-negotiation-program-next-steps-implementation-2026.pdf
9 “Key Dates and Estimated Timeline to Implement the Medicare Drug Price Negotiation Program for Initial Price Applicability Year 2026” Center for Medicare & Medicaid Services (Last accessed January 20,2023), https://www.cms.gov/files/document/medicare-drug-price-negotiation-program-next-steps-implementation-2026.pdf
10 Alice Miranda Ollstein, “‘The slippery slope is powerful’: Dems believe drug pricing law will pay dividends,” POLITICO Healthcare (December 29, 2022), https://www.politico.com/ news/2022/12/29/democrats-drug-pricing-medicare-00075246.
11 Rachel Roubein and McKenzie Beard, “Bernie, on his agenda for the Senate health panel,” The Washington Post, The Health 202 (January 11, 2023), https://www.washingtonpost.com/politics/2023/01/11/bernie-his-agenda-senate-health-panel/.
The views expressed in this article are those of the author(s) and not necessarily the views of FTI Consulting, its management, its subsidiaries, its affiliates, or its other professionals.
©2023 FTI Consulting, Inc. All rights reserved. www.fticonsulting.com



